In order to understand exergy, we must first understand energy. Energy is all around us, and it appears intuitively obvious to understand, yet it's difficult to define precisely. We still don't have a good definition of what energy is.
Energy is a scalar quantity that cannot be observed directly but can be evaluated indirectly by measuring how it changes form. It can take macroscopic forms dependent on reference frames, such as kinetic and potential energy, or microscopic forms independent of outside reference frames related to molecular structures and activity.
The First Law of Thermodynamics tells us that energy is always conserved in any transfer or transformation. The Second Law tells us that any spontaneous process occurs in the direction of increasing entropy, and all energy conversions are irreversible.
There is a relationship between entropy and the usefulness of energy. Energy is most useful to us when it is available to do work or when we can get it to flow from one place to another where we want it. Thus, useful energy must have low entropy so that the Second Law will allow transfer or conversions to occur.
Enter Exergy
Whenever a system varies in some way from its environment, in temperature, pressure, concentration, etc, it is not in equilibrium with its environment. This lack of equilibrium can be used to do work.
Exergy is the maximum work potential of a system in relation to a reference environment. It can be thought of as technical work capacity, usable energy, or the ability to do work.
More technically, the exergy of a system is defined as the maximum shaft work that can be done by a system when it is brought from its initial state to the dead state by means of processes involving interactions only with the reference environment.
The dead state is the state of a system when it is in thermal, mechanical, and chemical equilibrium with a conceptual reference environment.
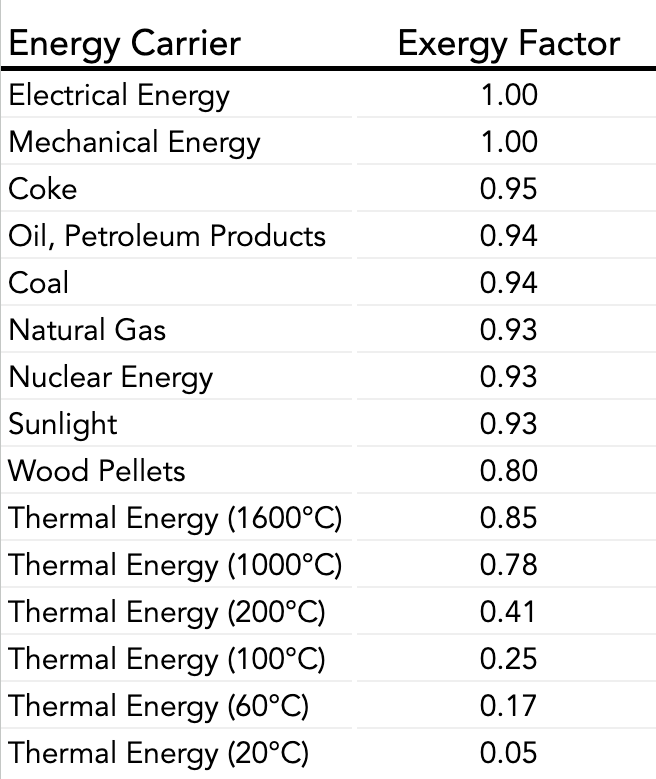
Energy that's high in entropy is low in exergy. Therefore, exergy is a description of the quality and usefulness of energy. The energy grade function is the ratio of exergy to energy for a stream or system.
Energy is always conserved, whereas exergy is destroyed when an irreversible process occurs – all real processes are irreversible. Exergy destructions represent losses in energy quality and usefulness. Exergy destruction is proportional to entropy generation (Gouy–Stodola theorem). Exergy efficiency is a measure of the nonideality or irreversibility of a process.
Exergy can be thought of as the degree of disequilibrium with the environment. A system has no exergy when it is in complete equilibrium with its environment. The more a system deviates from the environment, the more exergy it has. Therefore, exergy depends not only on the state of the system but also on the state of the environment.
The exergy of a system increases as the deviation of its state from that of the environment increases. For instance, hot water has a higher exergy content in winter than on a hot summer day, while a block of ice contains little exergy in winter but a significant quantity in summer.
Exergy is an important quantity for determining the value of a resource. There are both energy and nonenergy resources, and exergy can measure the value of both. An example of a nonenergy resource is a mineral deposit. The higher the mineral concentration in the ground compared to the rest of the environment, the higher the exergy content. Once that mineral is mined and purified, its exergy is increased further – although it takes exergy inputs in the mining and purification process.
To illustrate the difference between energy and exergy, imagine a mug of hot water. If you add some cold water to the mug, the temperature of the water mixture goes down. The total energy in the mug is the same because there's more water at a lower temperature. However, the exergy in the mug is now lower because an irreversible heat interaction occurred. Exergy has been destroyed, and the water will never spontaneously return to its original hot temperature.
Exergy and Economics
The economic value of energy varies enormously with its quality.
The exergy of a quantity of energy or a substance can be viewed as a measure of its usefulness, quality, or potential to cause change. When energy quality decreases, exergy is destroyed. Exergy is the part of the energy that is useful to society and has economic value, and is thus worth managing carefully.
We should avoid using energy at a significantly higher quality than is needed for a task. For example, it doesn't make economic or exergetic sense to heat a house with coke, when the high temperatures produced by burning coke in a blast furnace are required for melting iron ore to make steel. Houses don't need 3500°F blast furnaces to keep them at 70°F. Perhaps, though, the waste heat from the steel-making process can be used in a district energy system to heat hundreds of homes without the need for an additional heat source.
A heat pump that produces temperatures of about 110°F would be sufficient to heat a house on a winter day, but could not be used to make steel.
For purely economic cost reasons, it's beneficial to minimize exergy destruction in various processes at both the industrial and individual levels in pursuit of what we want. There are cost, logistic, and environmental benefits to maximizing utility value for a given amount of exergy destruction. See Entropy and Utility Value. The fields of thermoeconomics and exergoeconomics seek to do this.
For economic development to be sustainable:
it must satisfy the needs and wants of society,
it must be environmentally and economically benign, and
sufficient natural and human resources must be available.
People often talk colloquially about "energy conservation," "energy efficiency," and "saving energy" to achieve sustainability goals. These terms are misguided, since energy is always conserved. What's lost is exergy. When people talk about energy, often they really mean exergy, but don't know what it is. Understanding the difference between exergy and energy is important for optimizing economic and sustainable development.
Solar Exergy and Life
Pretty much all the energy delivered to Earth by electromagnetic radiation (light) from the sun is re-emitted to space as infrared radiation lower in exergy. The net change in energy to the Earth via the sun is zero, but overall the sun provides a net supply of exergy to the Earth. This exergy is gradually destroyed, but during its destruction, it manages to drive the Earth's water and wind systems and support life. Plants absorb exergy from sunlight and convert it via photosynthesis into chemical exergy. The chemical exergy then passes through different food chains in ecosystems, from microorganisms to people, powering all life on the planet.
"Rabbits have emerged as a pathway by which the universe degenerates and the quality of energy degrades. Rabbits, like primroses, pigs, and people, are part of the great network, the cosmic interconnection that allows temporary structures to emerge as degeneration ineluctably lowers the universe towards its final equilibrium." – Peter W. Atkins, The Second Law
Life requires exergy and catalyzes its destruction.
Exergy and Environmental Impact
One consequence of the Second Law of Thermodynamics is that all real processes must have some environmental impact. Exergy may be the most important link between the Second Law and understanding environmental impact.
The creation of chaos, or the destruction of order, is a form of environmental damage.
The observation that people are bothered by a landscape polluted with papers chaotically scattered about, but value the order of a clean field with the papers neatly piled at the side, suggests that, on a more abstract level, ideas relating to exergy and order in the environment may involve human values and that human values may in part be based on exergy and order. – W. Hafele, Energy in a Finite World
The degradation of natural resources is also a form of environmental damage. A resource can be defined as something that is in a state of disequilibrium with the environment.
The exergy associated with waste emissions presents a potential for environmental damage since the exergy of the wastes, as a consequence of not being in stable equilibrium with the environment, represents a potential to cause change.
One example of this is when a power plant uses water from a river to cool its steam turbine. The water it discharges back into the river is warmer than the rest of the water and contains exergy. This form of thermal pollution has the potential to disrupt local ecosystems, altering the exergy available to drive chemical reactions in living organisms.
Sometimes waste products have the potential to alter the natural exergy balance of the Earth by altering the exergy flow from the sun, as in the case of greenhouse gasses.
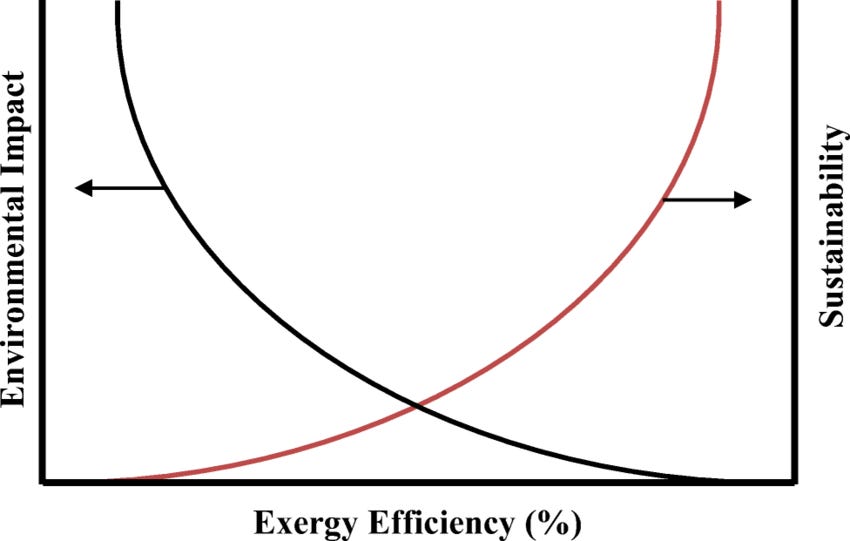
All resource use leads to some degree of environmental impact. There is a direct relation between exergy efficiency and environmental impact. An increase in efficiency means a fixed level of services can be satisfied with fewer energy resources and, in most instances, reduced levels of related waste emissions.
As exergy efficiency approaches 100%, environmental impacts approach zero because exergy is converted from one source to another without any consumptions or emissions. Sustainability approaches zero as exergy efficiency approaches zero because nothing is accomplished while exergy-containing resources are consumed.
Exergy efficiency optimization is a win-win because it minimizes the use of resources and decreases the cost of a given process while also minimizing the corresponding emissions and environmental damage.
Conclusion
Exergy describes the usefulness of energy and resources. It sits at the confluence of energy, the environment, and sustainable development. Exergy analysis allows engineers to aim for the highest possible energy and exergy efficiencies at the lowest costs while minimizing environmental disruption.
Questions for you:
What is one way exergy destruction can be minimized in your life while maintaining the same level of utility value?
What is one way exergy destruction can be minimized in our economy while maintaining the same level of utility value?
How does the concept of exergy change how you think about energy?





Great article! I learned some key ideas here. Thank you Tanner!